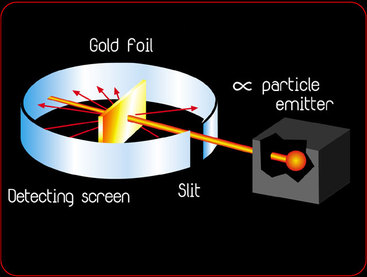
Rutherford's Gold-Foil Experiment
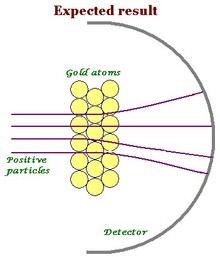
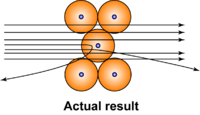
Onion the Omniscientist: Well... According to the Plum Pudding Model, it was expected that they would mostly pass directly through the gold foil with little or no deflection. Instead, they noticed that although the majority of the alpha particles did just that, there were actually some alpha particles that either deflected in the direction it came from or at really large angles.
Onion the Omniscientist: Rutherford actually figured out quite a few things. He inferred that there must be very strong particles that reflected the alpha particles. Yet, they had to be extremely small since only a tiny fraction of the alpha particles were affected by it. He also inferred that the area of these particles had to be densely compacted with a positive charge, which he named the nucleus.