From Atomic Theory to Plum Pudding Model
Onion the Omniscientist: Hi there, Clueless Cat! Let's start from the beginning! Our best “picture” of the atom changes as new discoveries are made, and our models have to be modified to encompass these discoveries. John Dalton's Atomic Theory stated that atoms were indivisible, which you know is no longer supported in the best picture of the atom.
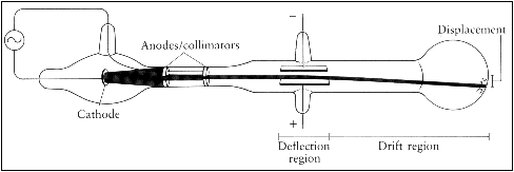
Onion the Omniscientist: Well, Clueless Cat, I'll give you a hint: It was a result of William Crooke's conclusion that cathode rays (rays given off by a metal plate connected to the negative terminal of a voltage source) are negatively charged by observing their deflection by a magnetic field, which has a negative charge as well. So what do you think?
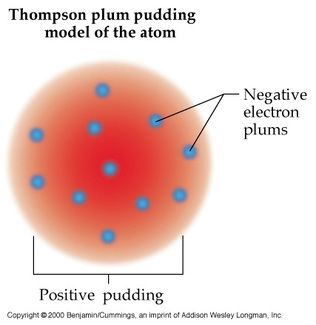
Onion the Omniscientist: Well, J.J. Thompson’s experiments led to the conclusion that particles make up matter. Through an experiment, he discovered that cathode rays were actually particles, later to be known as electrons. (An electron is a fundamental negatively charged unit.) Since atoms must contain negatively charged electrons, it must contain positively charged particles because atoms are electrically neutral, which must also account for the rest of the atom's mass.
To continue on this wonderful atomic model discovery,