Rutherford-Bohr Model
Onion the Omniscientist: He had to invite Bohr to work on his atomic model because classical theory suggested that the atoms would have a short lifespan. He noticed another problem that contradicted classical theory was the emission spectrum of a hydrogen atom, which he incorporated into the Rutherford-Bohr Model.
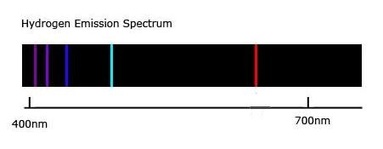
Onion the Omniscientist: Classical theorists hypothesized that any amount of energy could be added to a hydrogen atom, and therefore, they expected hydrogen gases' refractions through a prism to emit continuous spectrums. Hydrogen's line-emission spectrum showed that only specific energy states of electrons can exist in hydrogen atoms. So Bohr came up with orbits, which represented energy states that electrons can’t exist between.
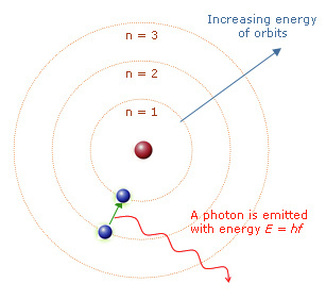
Onion the Omniscientist: Although the whole word is in it, they are not the same! Rutherford and Bohr represented orbits as an electron's path around the nucleus, which increase in energy further away from the nucleus. The Rutherford-Bohr model was the most recent model of the atom at that time but it would break the Heisenburg Uncertainty Principle and it doesn’t even work for bigger atoms!
Onion the Omniscientist: Yes! Because the Rutherford-Bohr model only described the size of the orbits with one quantum number, n, which you have learned is not enough to describe even an orbital. But as of now, that is all I have to say about the Evolution of the Atomic Model, but you are not as clueless as they say!